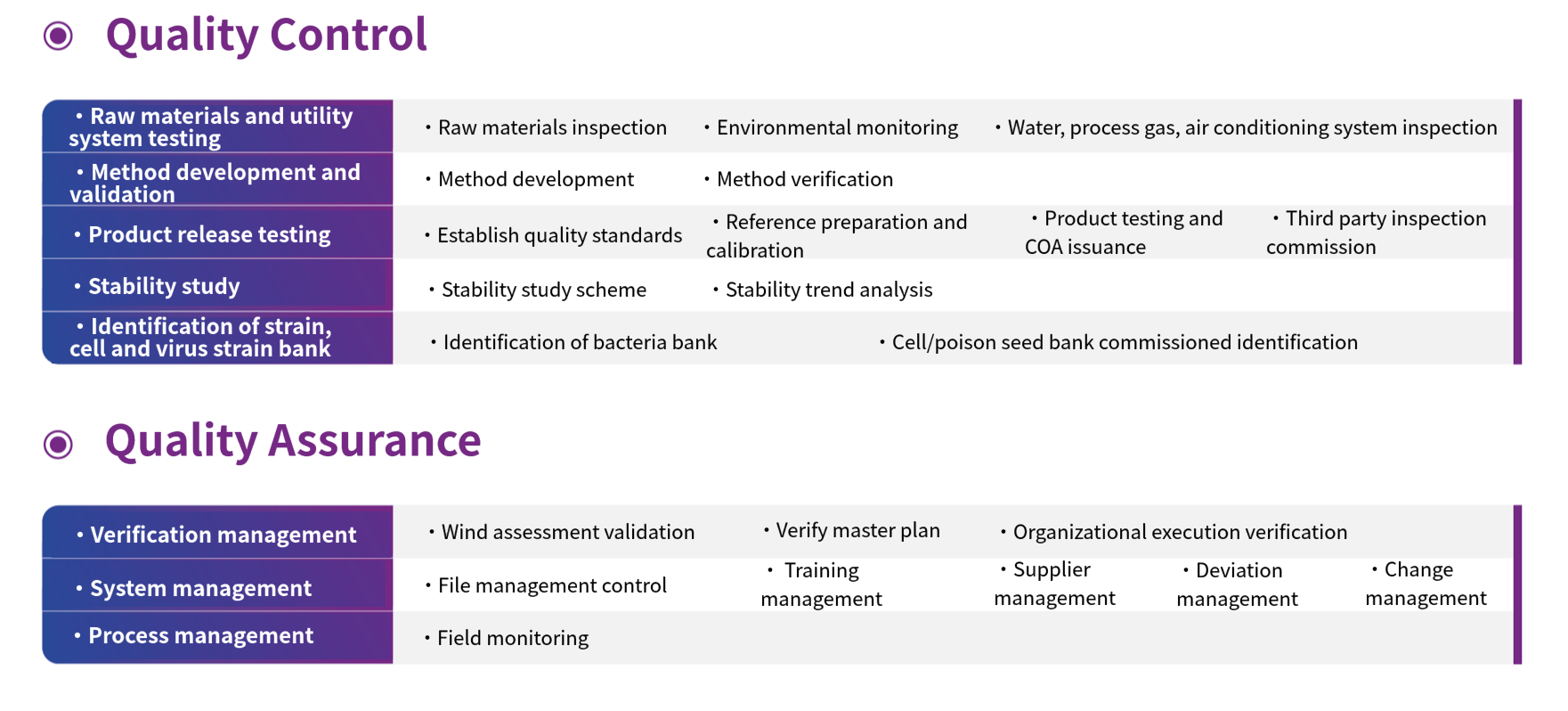
Quality Control and Assurance System

Based on the platform's comprehensive quality management system, quality control methods have been established for different physicochemical properties of products, implementing comprehensive process control and drug quality release management, with quality system construction running through the entire QC inspection process.
- Detection of raw materials and utility systems
- Identification of strain, cell, and toxin banks
- Stability studies
- Method development and testing
- Product release testing
LotusLake BioMedical manages its operations according to the requirements of various departments and business needs, in compliance with regulations such as ISO, GLP, and GMP. We are equipped with professional QA personnel who are responsible for comprehensive quality management to meet our clients' quality standard requirements for GLP and GMP.
- Validation management
- Quality assessment
- Process supervision
- Documentation and training
- QMS (Quality Management System)